no3 molar mass|al no3 3 compound name : Manila There are 4 easy steps to find the molar mass of NO3 {-} based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Nitrate Ion . WEBPorky Payout review, a detailed look into Microgaming's Video Slot game Porky Payout including relevant casino bonuses, payouts, game features and screenshots.
0 · what is no3 in chemistry
1 · what is no3 called
2 · what does no3 2 mean
3 · ni no3 2 compound name
4 · how is molar mass calculated
5 · equivalent mass of no3
6 · al no3 3 compound name
7 · al no3 3 chemical name
8 · More
Fortune Tiger é uma caça-níqueis que combina sorte e estratégia para maximizar seus ganhos. Aqui estão algumas táticas eficazes para aumentar suas chances de vitória. Ver mais
no3 molar mass*******There are 4 easy steps to find the molar mass of NO3 {-} based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Nitrate Ion .
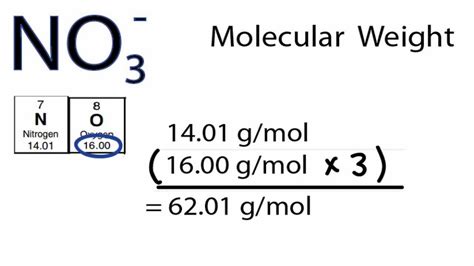
Molar mass is the mass (in atomic mass units) of one mole of a of a substance. . Learn how to calculate the molar mass of a substance by summing the molar masses of its component atoms. See examples, questions, and tips on how to convert between mass .
Find the molar mass of NO3, a nitrate ion, and its percentage composition by element. Learn how to calculate molar mass and molecular weight from atomic w. Learn how to calculate the molar mass of NO3-, the nitrate ion, using the periodic table and the subscripts in the formula. Watch a video explanation with examples and tips.In the NO−3 anion, the oxidation state of the central nitrogen atom is V (+5). This corresponds to the highest possible oxidation number of nitrogen. Nitrate is a potentially powerful oxidizer as evidenced by its explosive behaviour at high temperature when it is detonated in ammonium nitrate (NH4NO3), or black powder, ignited by the shock wave of a primary explosive. However, in contrast to red fuming nitric acid (HNO3/N2O4), or concentrated nitric acid (HNO3), nitrate dissolved in aqueous solutionLearn how to calculate the molar mass and molecular weight of NO3 (nitrate radical) and its elemental composition. Find the chemical structure, appearance, reactions and .Molar mass is the mass (in atomic mass units) of one mole of a of a substance. One atomic mass unit (u) is equal to 1/12 the mass of one atom of carbon-12. It is also sometimes .NITRATE ION is a chemical compound with the formula NO3-. Its average mass is 62.005 Da and its monoisotopic mass is 61.988365 Da. Find more names, properties, spectra, vendors and articles on .
Learn how to calculate the molar mass of NO3 by adding the molar masses of nitrogen and oxygen atoms. Watch a one-minute video and see examples of . Calculate the molar mass of any element or compound using the atomic masses of its constituents. Learn the definition, formula and examples of molar mass .
no3 molar mass al no3 3 compound nameSolution. First we need to determine the mass of one mole of methane (CH 3 OH). Using the periodic table to find the mass for each mole of our elements we have: 1moleC = 1 moleC ×(12.011gC 1 moleC) = 12.011gC (8.3.8) (8.3.8) 1 m o l e C = 1 m o l e C × ( 12.011 g C 1 m o l e C) = 12.011 g C.al no3 3 compound nameThe nitrate ion with the partial charges shown. The nitrate anion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal .The molar mass of a compound defines the mass of 1 mole of that particular substance and number of grams per mole of a compound. . Therefore, the molar mass of Ca(NO3)2 is 164.1 g/mol. Note that the subscript two after the parentheses specifies that there are 2 nitrate ions (NO3-).
Finally, add together the total mass of each element to get the molar mass of PB((NO3)2): 30.973762 g/mol + 10.811 g/mol + 28.0134 g/mol + 95.9964 g/mol = 165.794562 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in PB((NO3)2), divide each total from step 3 by the total molar mass found in step 4:The molar mass of any compound is the mass in grams of one mole of that compound. One mole of carbon dioxide molecules has a mass of 44.01g 44.01 g, while one mole of sodium sulfide formula units has a mass of 78.04g 78.04 g. The molar masses are 44.01g/mol 44.01 g/mol and 78.04g/mol 78.04 g/mol respectively. In both cases, that is .3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in AgNO3: Molar Mass (g/mol) Ag (Silver) 1 × 107.8682 = 107.8682. N (Nitrogen) 1 × 14.0067 = 14.0067.Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in (NO3)2: Molar Mass (g/mol) N (Nitrogen) 2 × 14.0067 = 28.0134. O (Oxygen) 6 × 15.9994 = 95.9964.14.0067. O (Oxygen) 15.9994. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in 2Mg (NO3)2: Molar Mass (g/mol) Mg (Magnesium) 2 × 24.305 = 48.61.
Finally, add together the total mass of each element to get the molar mass of Fe (NO3)3: 55.845 g/mol + 42.0201 g/mol + 143.9946 g/mol = 241.8597 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in Fe (NO3)3, divide each total from step 3 by the total molar mass found in step 4:15.9994. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Ba ( (NO3)2): Molar Mass (g/mol) Ba (Barium) 1 × 137.327 = .3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in In (NO3)3: Molar Mass (g/mol) In (Indium) 1 × 114.818 = 114.818. N (Nitrogen) 3 × 14.0067 = 42.0201.3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Zn (NO3)2: Molar Mass (g/mol) Zn (Zinc) 1 × 65.409 = 65.409. N (Nitrogen) 2 × 14.0067 = 28.0134.
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Cu (NO3)2: Molar Mass (g/mol) Cu (Copper) 1 × 63.546 = 63.546. N (Nitrogen) 2 × 14.0067 = 28.0134.Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of NO3 (Nitrate radical) is 62.0049 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between NO3 .Nitrate (NO3 {1-}) molar mass. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of Nitrate (NO 3 {1-}) is 62.0054 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between NO3{1-} weight and moles.
15.9994. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in K (NO3): Molar Mass (g/mol) K (Potassium) 1 × 39.0983 = 39.0983. N (Nitrogen) 1 . Explanation of how to find the molar mass of Mg(NO3)2: Magnesium nitrate.A few things to consider when finding the molar mass for Mg(NO3)2:- make sure you ha.

3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in 2PB (No3)2: Molar Mass (g/mol) P (Phosphorus) 2 × 30.973762 = 61.947524. B (Boron) 2 × 10.811 = 21.622.3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in 2PB (No3)2: Molar Mass (g/mol) P (Phosphorus) 2 × 30.973762 = 61.947524. B (Boron) 2 × 10.811 = 21.622.
Castanho. Altura. 1,56 cm. Gabriel Breier ou simplesmente Breier, é um filho da puta youtuber famoso por espalhar sua piroca e estética para outras pessoas através das redes sociais, incluindo o ONLY FANS. História. ele fala que e hetero mas todo mundo sabe que ele e gay. Categorias.
no3 molar mass|al no3 3 compound name